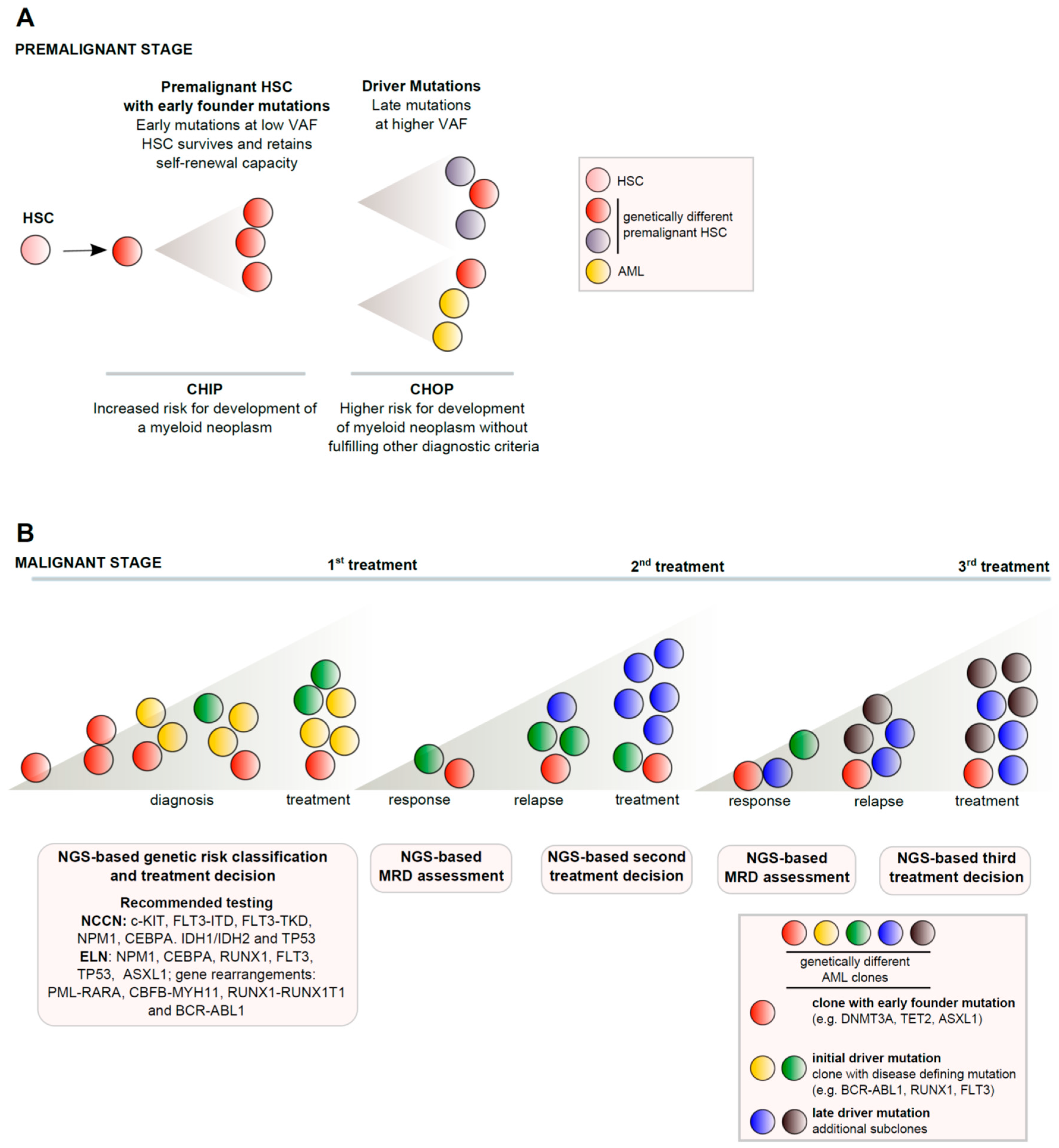
Cancers | Free Full-Text | Next Generation Sequencing in AML—On the Way to Becoming a New Standard for Treatment Initiation and/or Modulation?
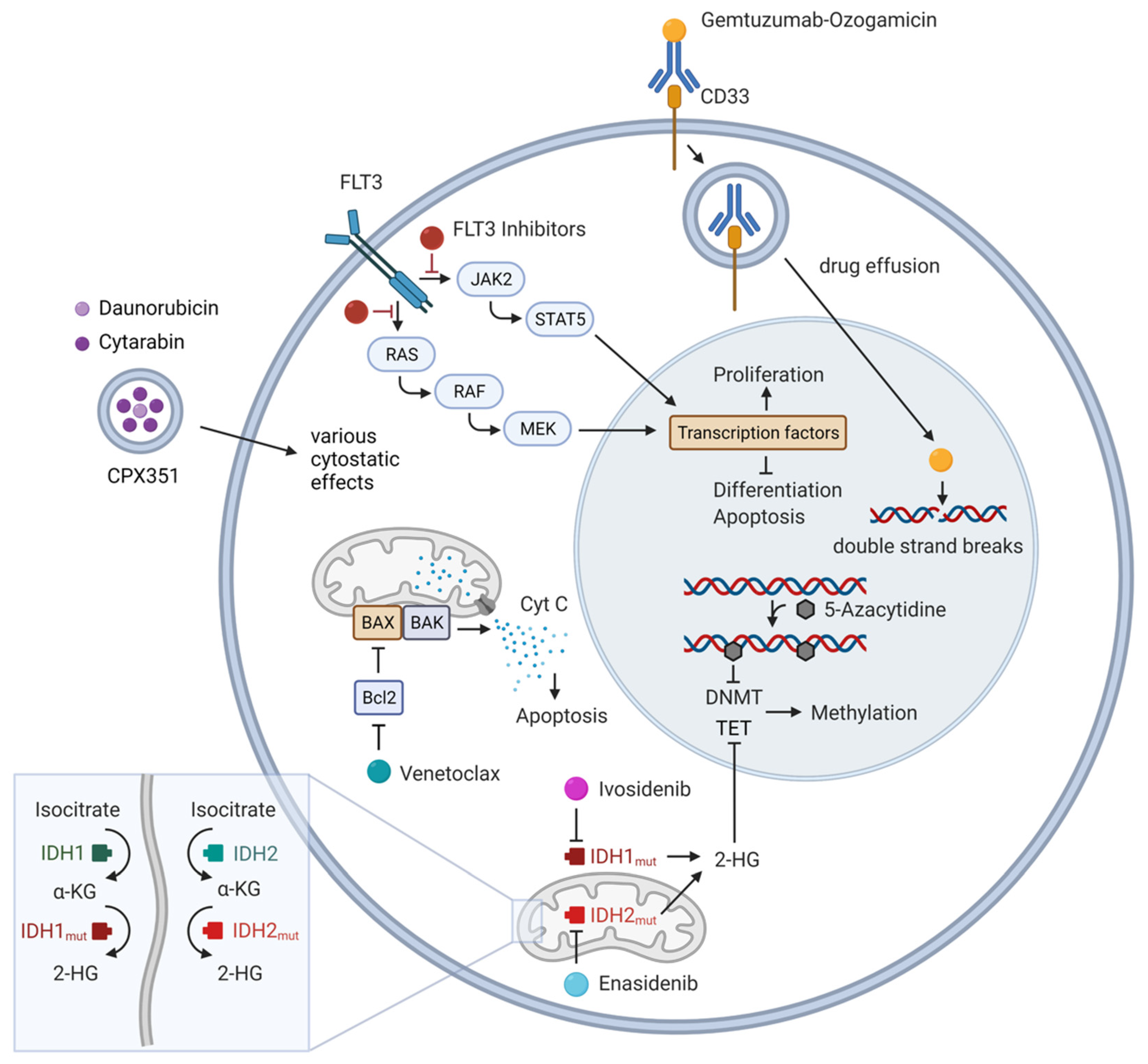
Cancers | Free Full-Text | Refining AML Treatment: The Role of Genetics in Response and Resistance Evaluation to New Agents

A review of the evidence base for utilizing Child-Pugh criteria for guiding dosing of anticancer drugs in patients with cancer and liver impairment - ScienceDirect