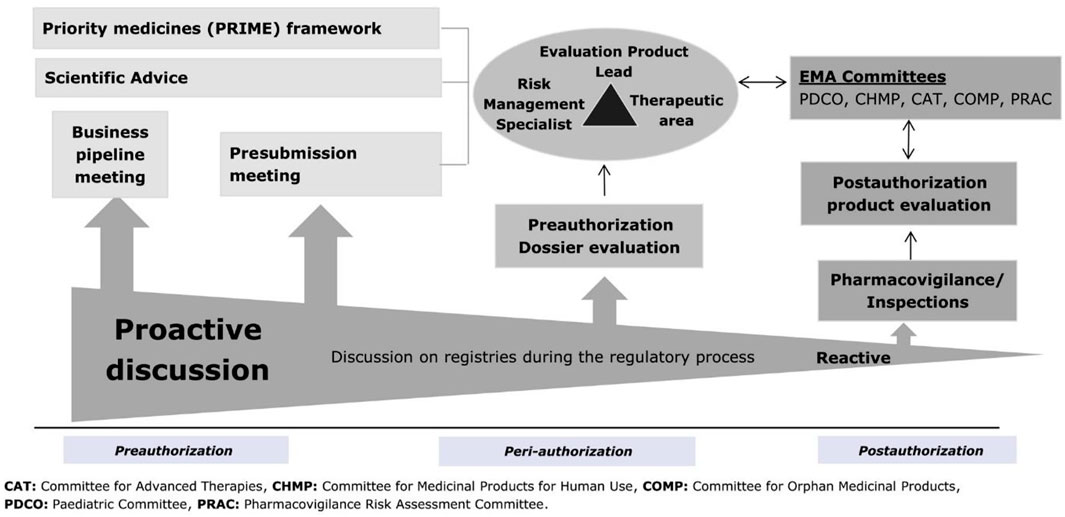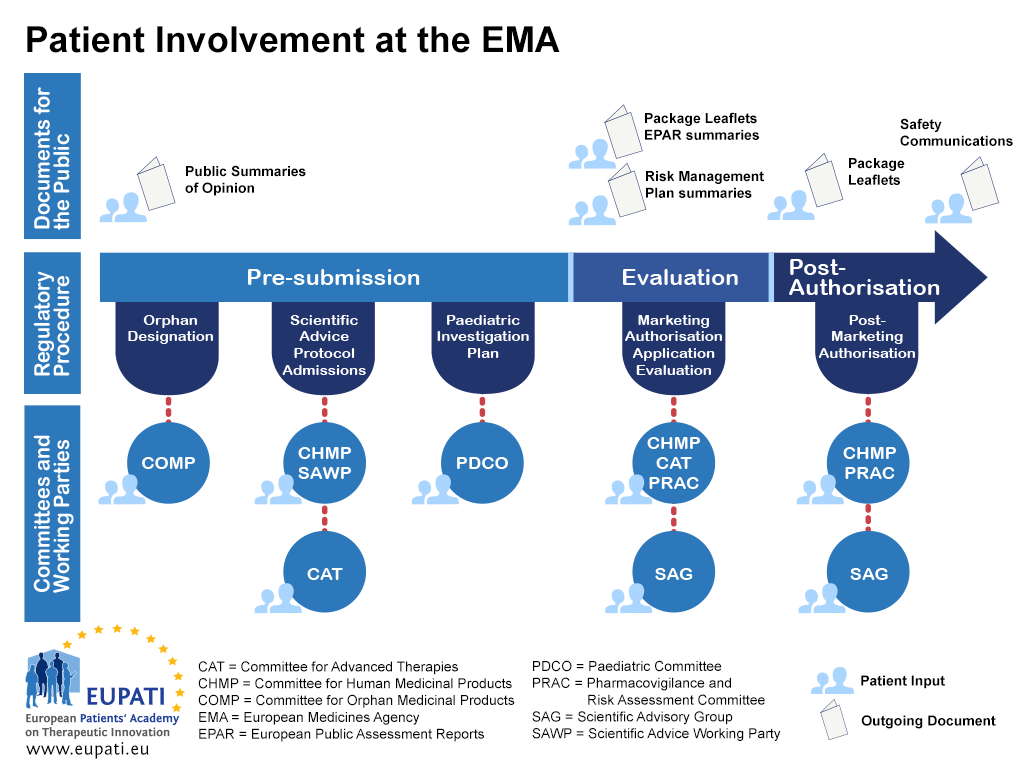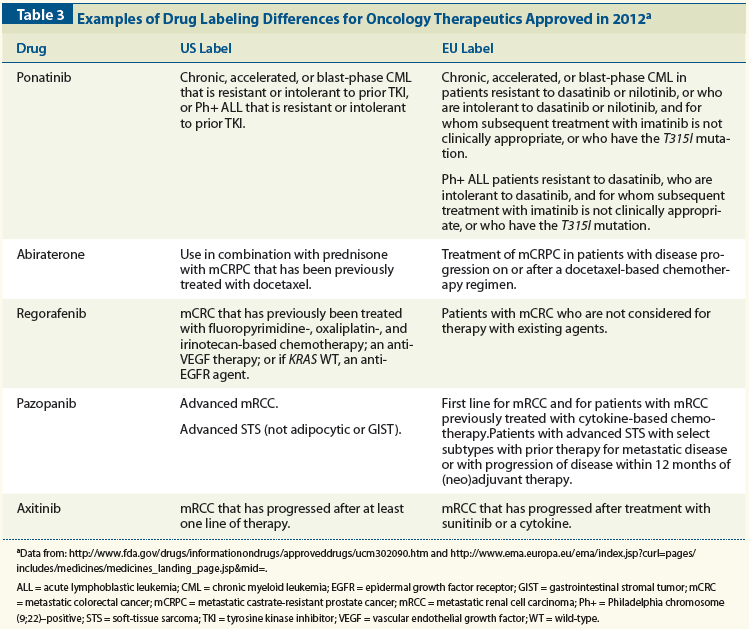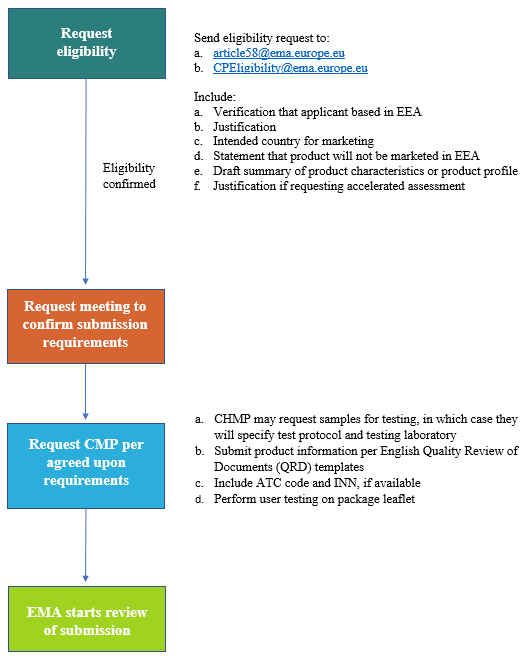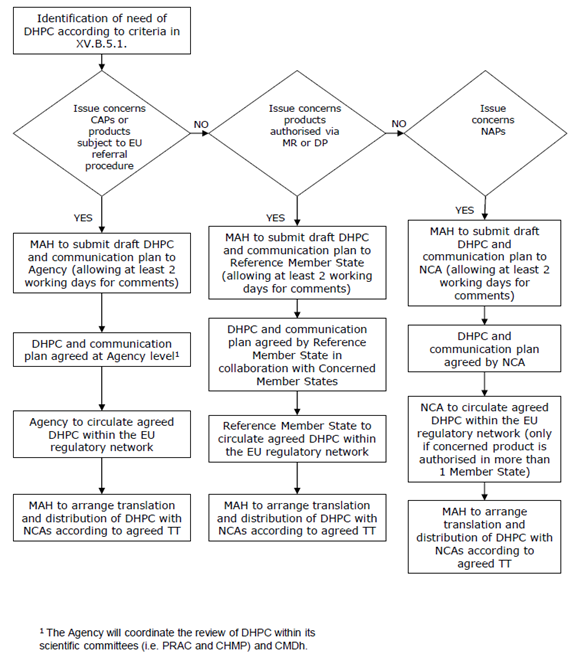
6. Direct healthcare professional communication. Communicating medicines safety information directly to healthcare professionals: an introduction to DHPC

Differences in Evidentiary Requirements Between European Medicines Agency and European Health Technology Assessment of Oncology Drugs—Can Alignment Be Enhanced? - Value in Health

Pharmaceutics | Free Full-Text | The Biosimilar Landscape: An Overview of Regulatory Approvals by the EMA and FDA
Quality Review of Documents veterinary product-information annotated template (English) version 8 - clean

Kühler et al 2019 – To what degree are review outcomes aligned for new active substances between the EMA and the US FDA? – CIRS

Impact of the European Union on access to medicines in low- and middle-income countries: A scoping review - The Lancet Regional Health – Europe

Guidelines for Emergency Managers working with Culturally and Linguistically Diverse Communities - World | ReliefWeb

Medicines for Europe Press release – EMA states no scientific evidence to deter use of ibuprofen in COVID19 patients | Medicines for Europe