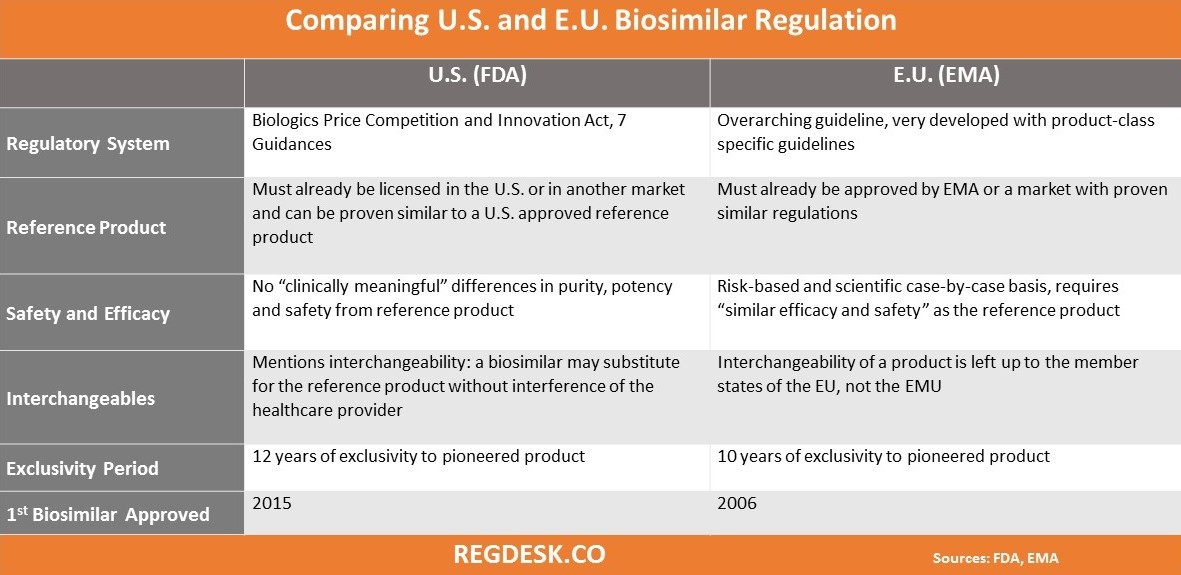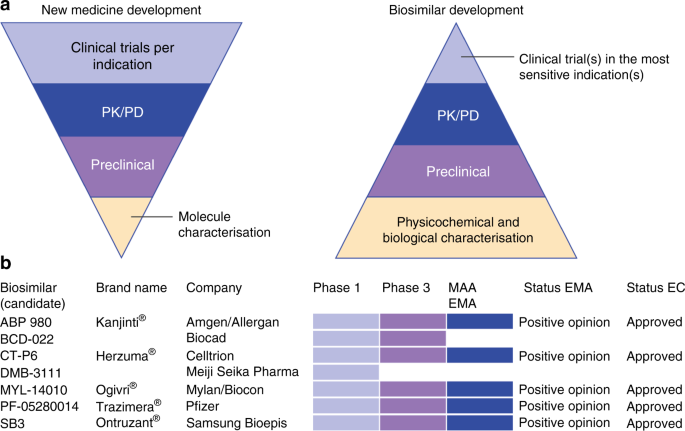
The arrival of biosimilar monoclonal antibodies in oncology: clinical studies for trastuzumab biosimilars | British Journal of Cancer

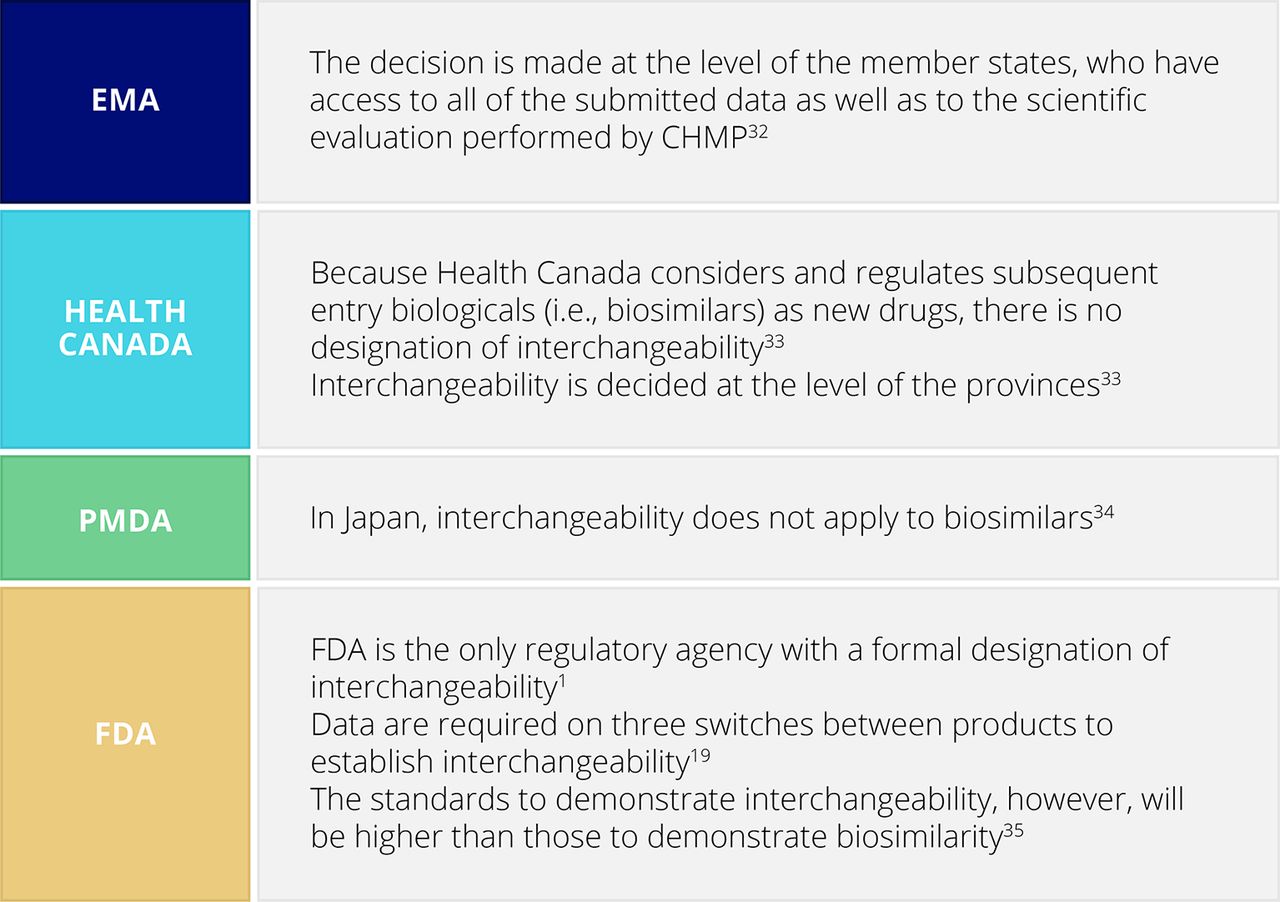
Pharmaceuticals | Free Full-Text | Analysis of the Regulatory Science Applied to a Single Portfolio of Eight Biosimilar Product Approvals by Four Key Regulatory Authorities

Sandoz on Twitter: "#DYK? The EMA leads the way with the longest list of approved #biosimilars, versus other highly-regulated markets. 43 biosimilar medicines are currently approved in to treat conditions in #oncology, #

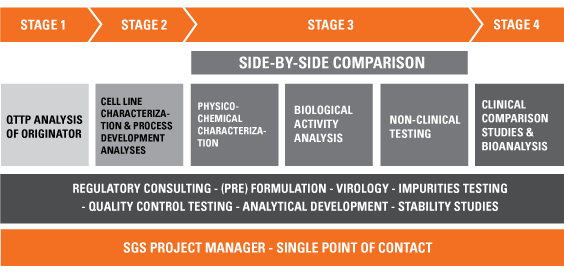
European Medicines Agency (EMA) approval processes for originator and... | Download Scientific Diagram
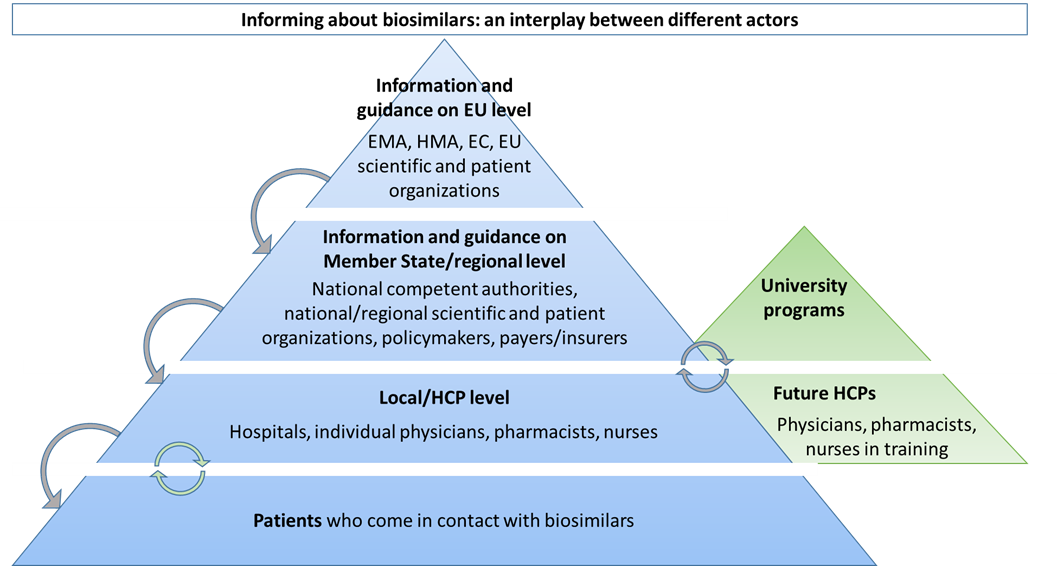
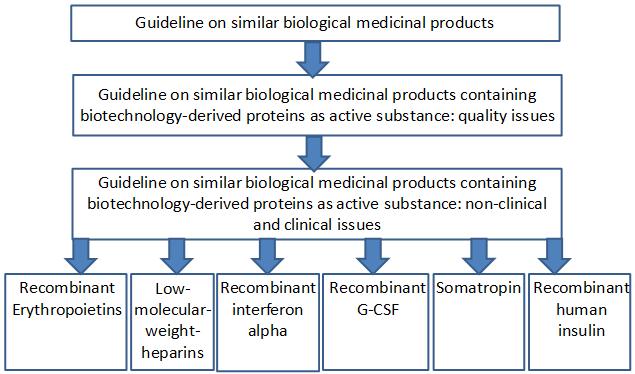
Effectively Educating Clinicians And Patients On Biosimilars Across Europe Getting The Right Message Across