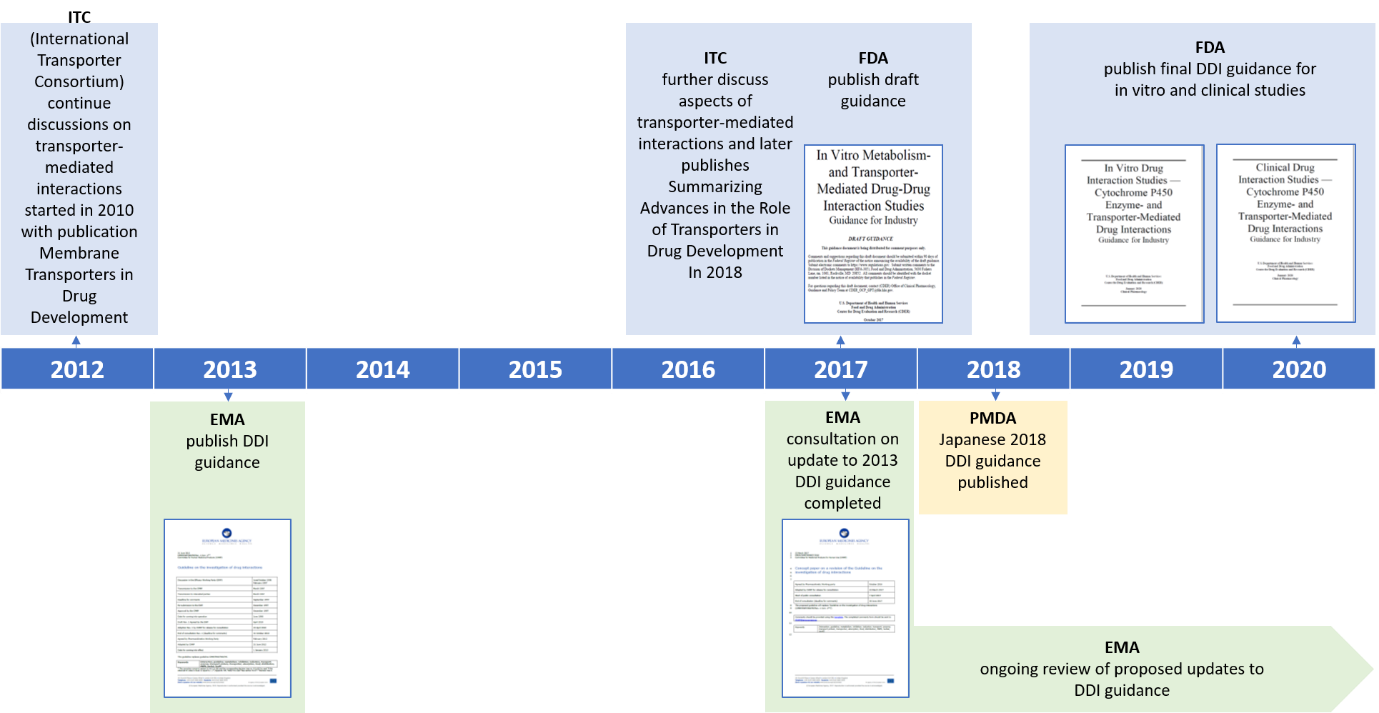
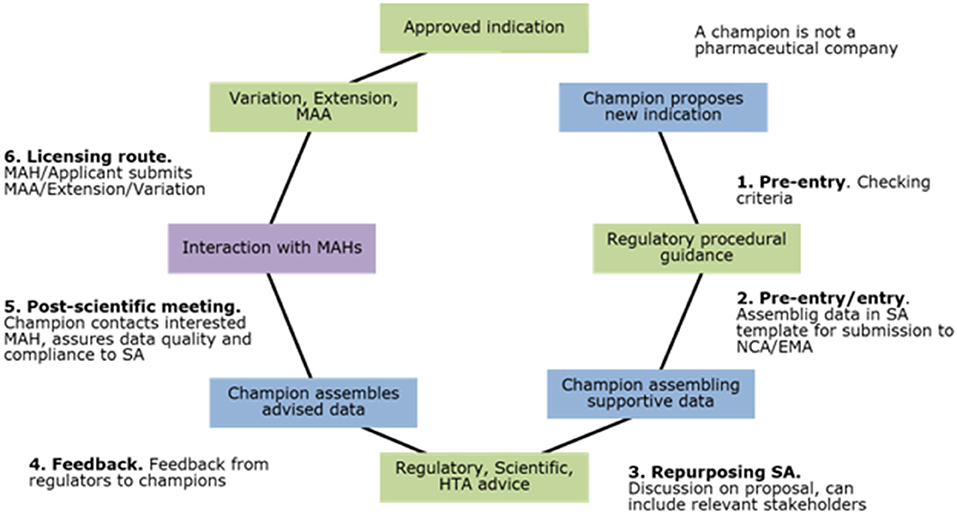
Biomarkers: Opportunities and Challenges for Drug Development in the Current Regulatory Landscape - Mariya Gromova, Annegret Vaggelas, Gabriele Dallmann, Diane Seimetz, 2020

A Decade of FDA-Approved Drugs (2010–2019): Trends and Future Directions | Journal of Medicinal Chemistry

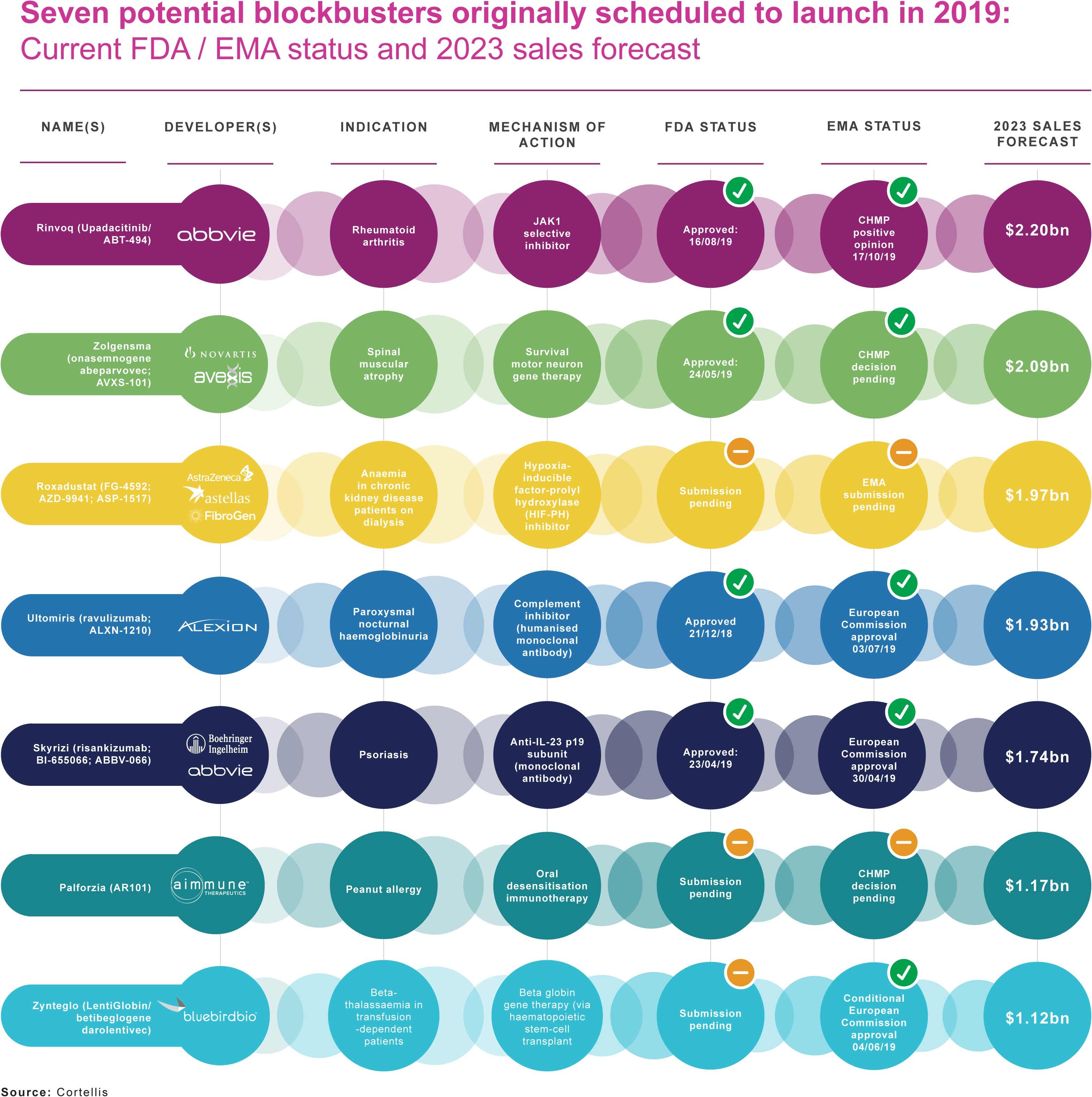
FDA Approves Four Biosimilars During Summer 2019 While Europe is on Vacation | Biosimilars Law Bulletin

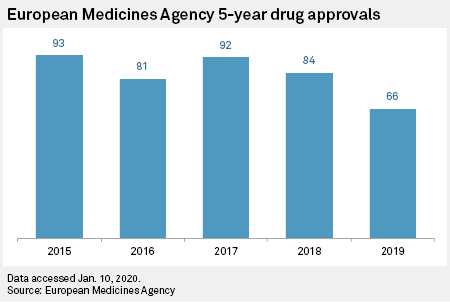
Association between FDA and EMA expedited approval programs and therapeutic value of new medicines: retrospective cohort study | The BMJ

An overview of cancer drugs approved through expedited approval programs and orphan medicine designation globally between 2011 and 2020 - ScienceDirect

A Decade of FDA-Approved Drugs (2010–2019): Trends and Future Directions | Journal of Medicinal Chemistry

Clinical Benefit and Expedited Approval of Cancer Drugs in the United States, European Union, Switzerland, Japan, Canada, and Australia | JCO Oncology Practice

FDA Approves Four Biosimilars During Summer 2019 While Europe is on Vacation | Biosimilars Law Bulletin
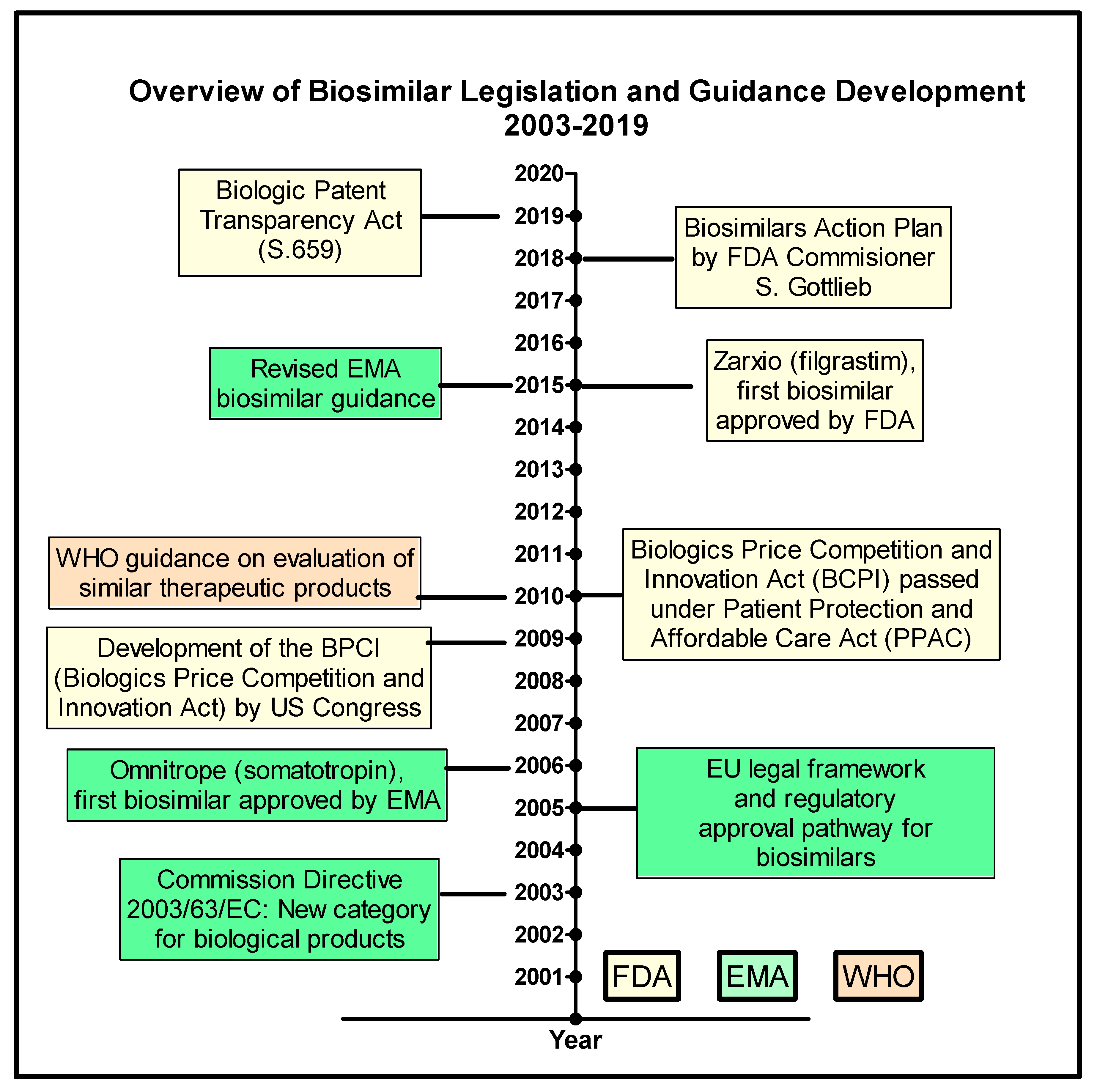
Pharmaceutics | Free Full-Text | The Biosimilar Landscape: An Overview of Regulatory Approvals by the EMA and FDA

From labs to shelves: EMA's new booklet explains how drugs are developed and marketed in the EU – TIF
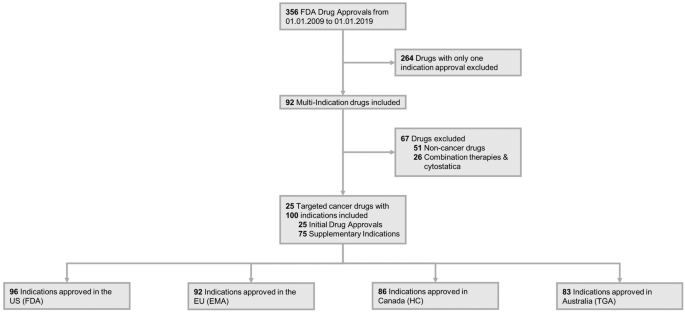
Initial and supplementary indication approval of new targeted cancer drugs by the FDA, EMA, Health Canada, and TGA | SpringerLink