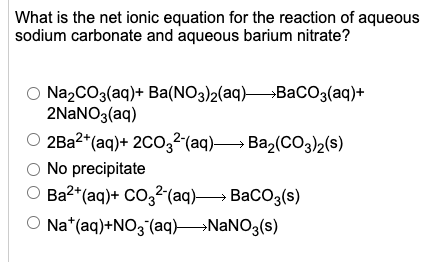
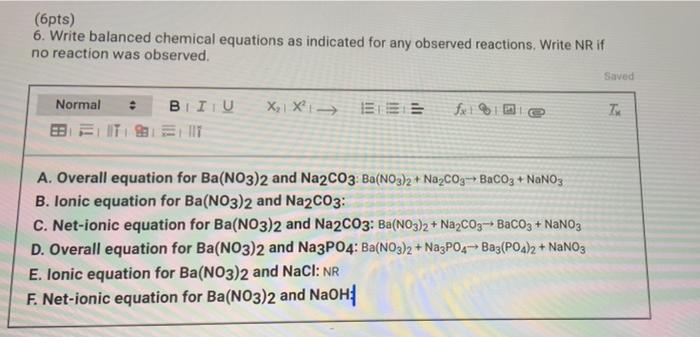
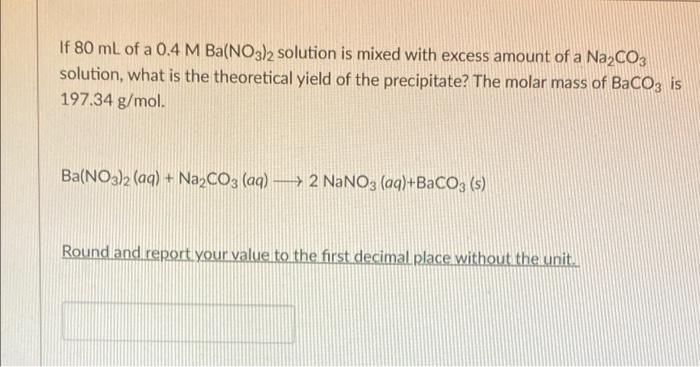
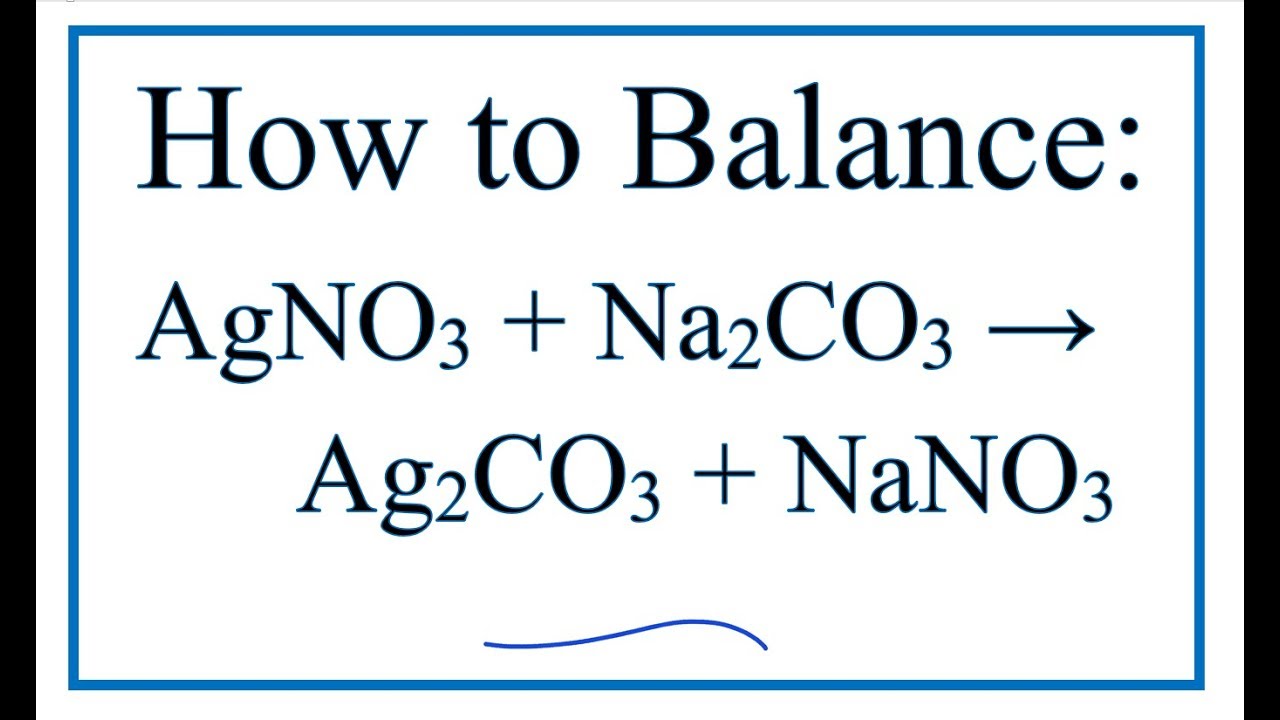
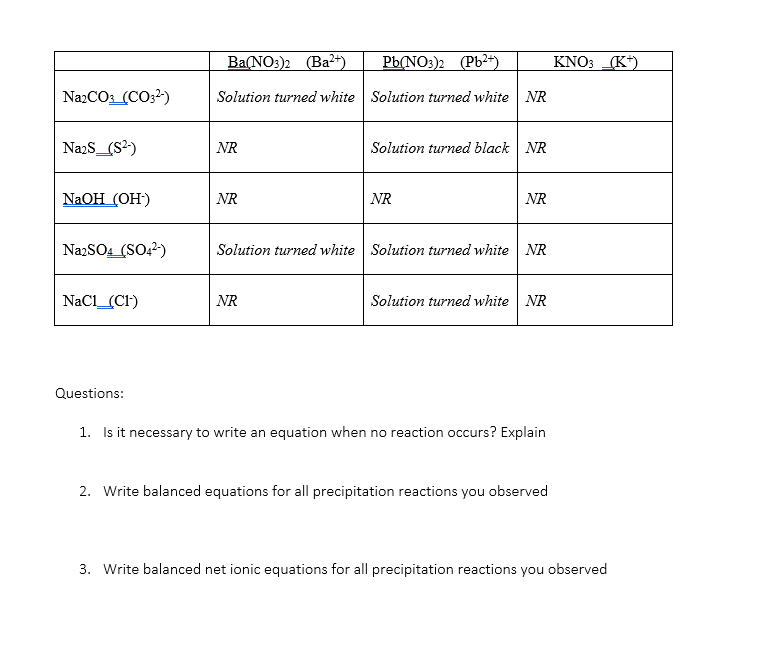
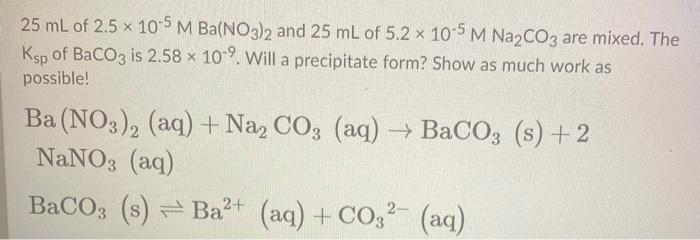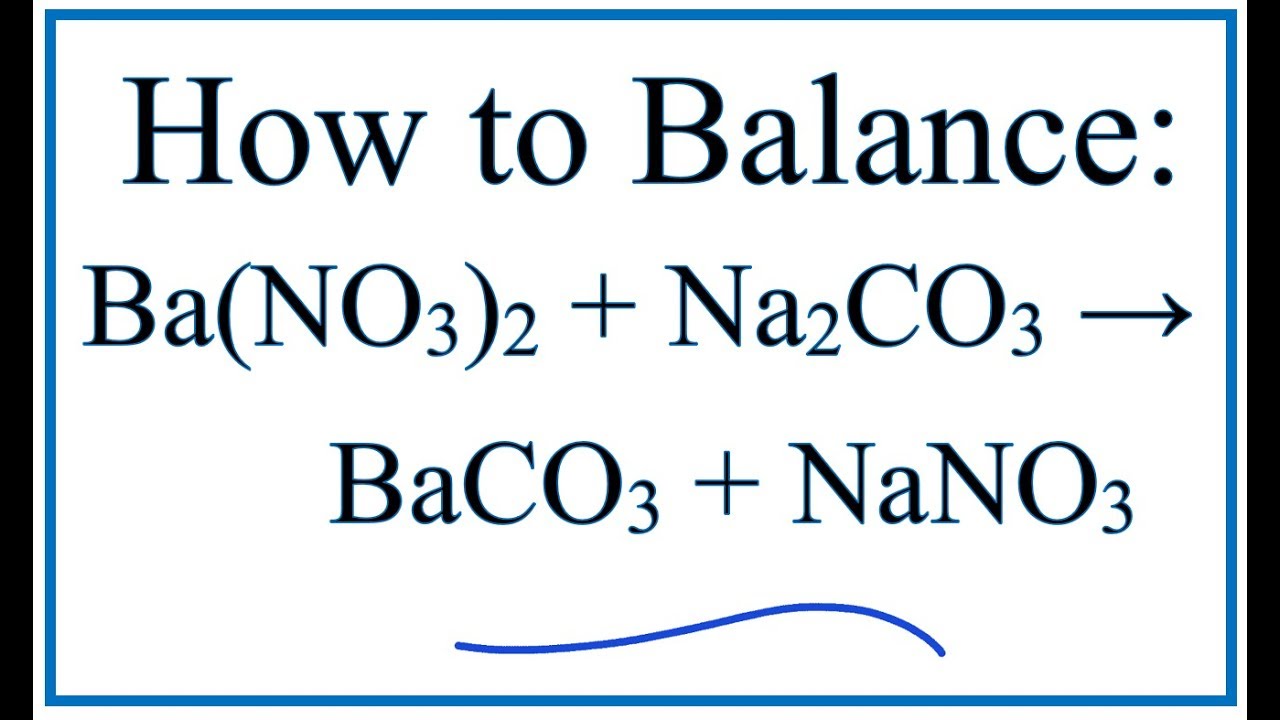Solid Ba(NO3)2 is gradually dissolved in a 1.0 × 10^-4 M Na2CO3 solution. At what concentration of Ba^2 + will a precipitate begin to form? ( KSP for BaCO3 = 5.1 × 10^-9 )

Solid Ba(NO3)2 is gradually dissolved in a 1.0 × 10^-4 M Na2CO3 solution. At what concentration of Ba^2 + will a precipitate begin to form? ( Ksp for BaCO3 = 5.1 × 10^-4 )
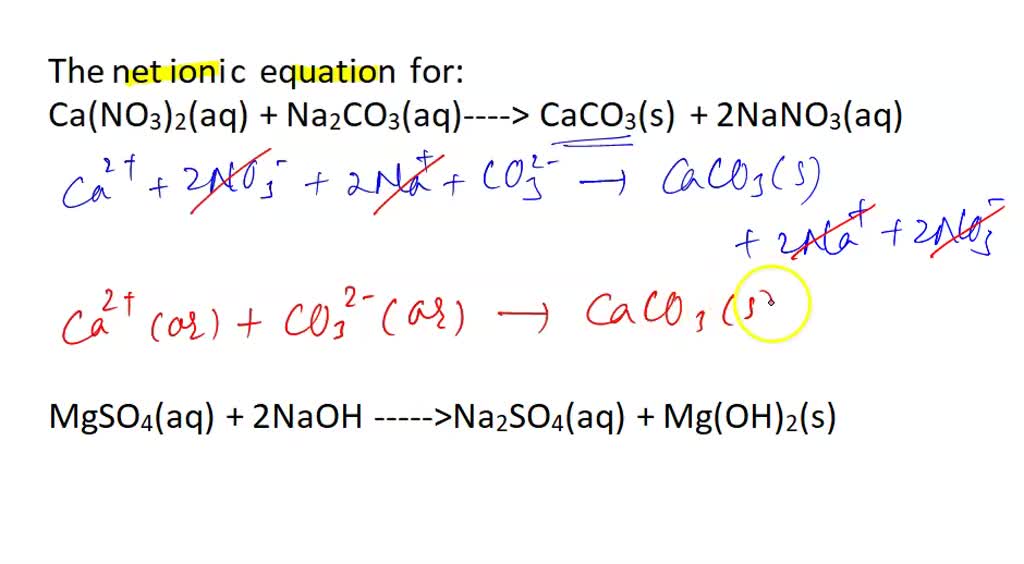
SOLVED: The net ionic equation for: Ca(NO3)2(aq) + Na2CO3(aq ->CaCO3(s) + 2NaNO3(aq) MsSO4(aq) + 2NaOH ->Na2SO4(aq) + Mg(OH)2(s) NaNO3(aq) + KCl(aq) ->KNO3(aq) + NaCl(s) HCl(aq) + NaOH(aq) -> NaCl(aq) + H2O(l)
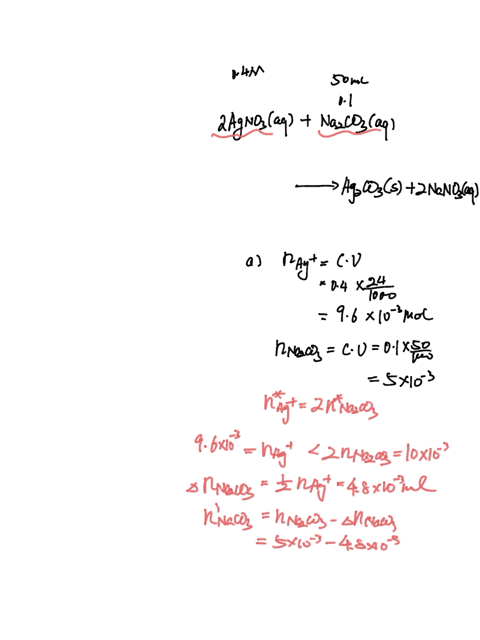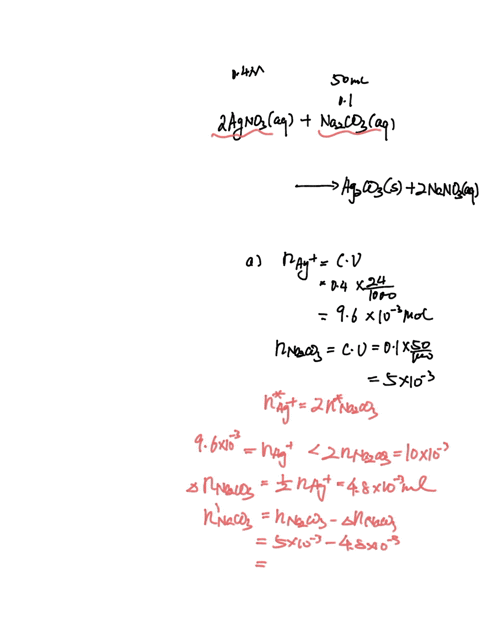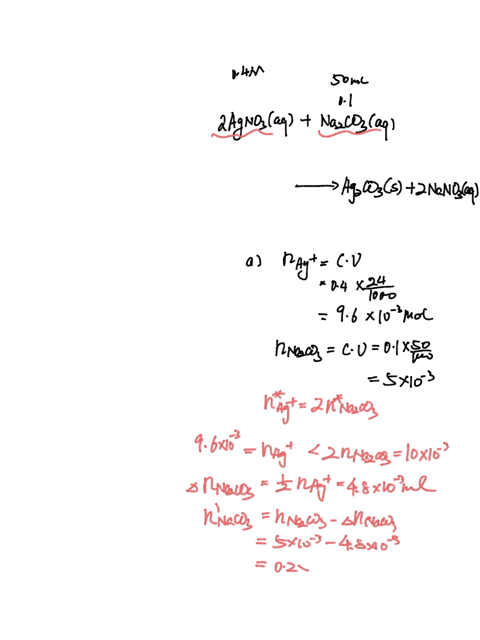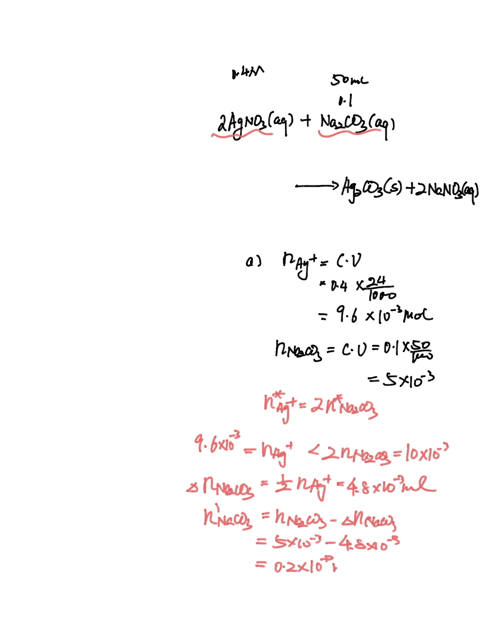
SOLVED: A sample of 20.0mL of 0.10M Ba(NO3)2 is added to 50.0mL of 0.10M Na2CO3. You wish to determine whether BaCO3 (Ksp = 8.1 x 10-9) will precipitate. The necessary calculation for