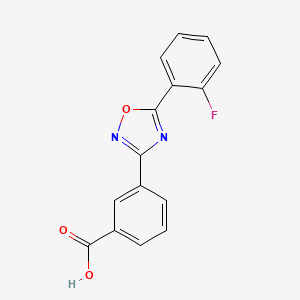
Ataluren use in patients with nonsense mutation Duchenne muscular dystrophy: patient demographics and characteristics from the STRIDE Registry
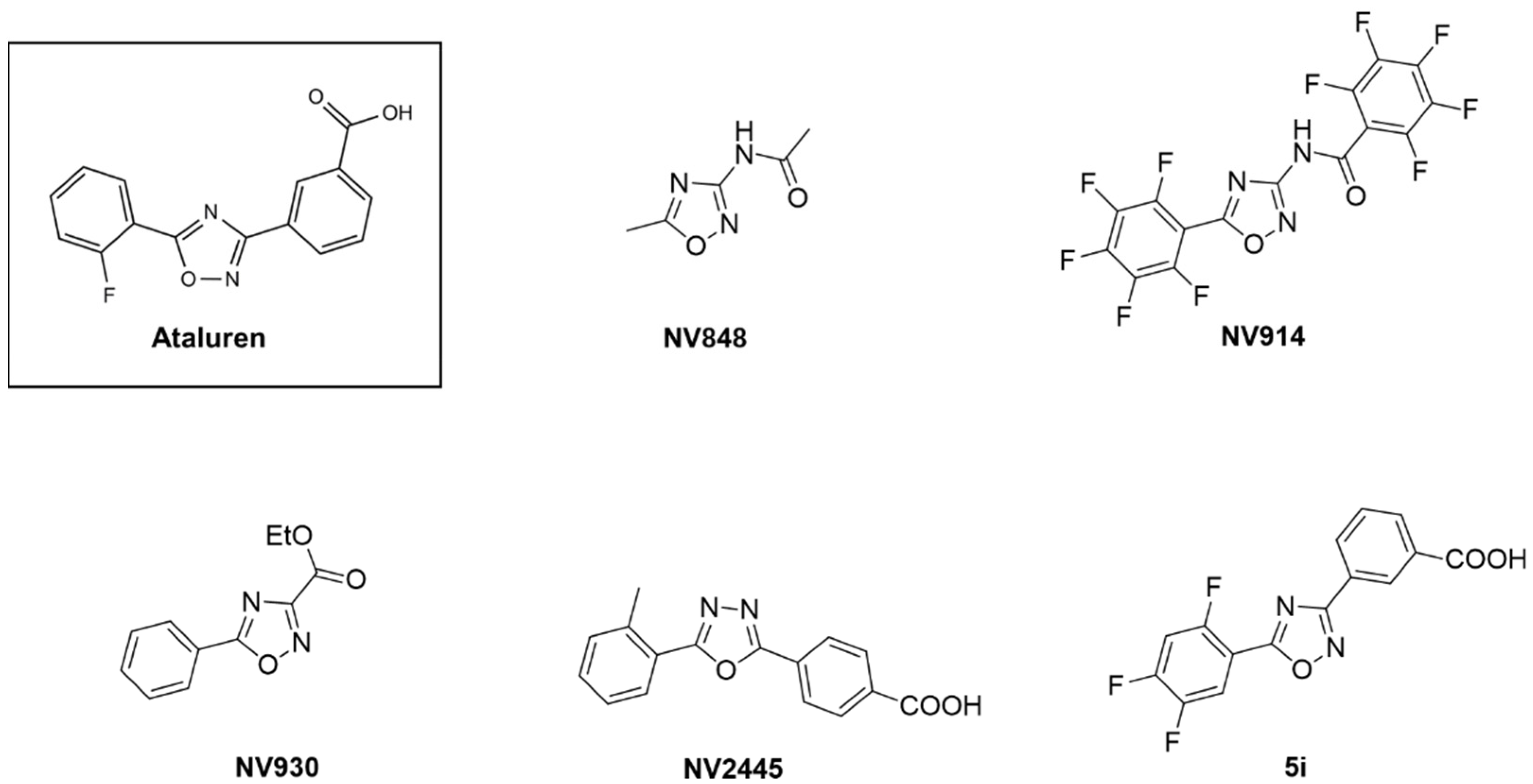
Biomedicines | Free Full-Text | Novel Translational Read-through–Inducing Drugs as a Therapeutic Option for Shwachman-Diamond Syndrome
Translarna (ataluren) for the Treatment of Nonsense Mutation Duchenne Muscular Dystrophy (nmDMD) - Clinical Trials Arena
Ataluren: An Investigational Dystrophin Restoration Drug for Nonsense Mutation Duchenne Muscular Dystrophy

Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial - The Lancet
Translarna (ataluren) for the Treatment of Nonsense Mutation Duchenne Muscular Dystrophy (nmDMD) - Clinical Trials Arena

Meta-analyses of ataluren randomized controlled trials in nonsense mutation Duchenne muscular dystrophy

DMD Aileleri Derneği on Twitter: "PTC Therapeutics, non-sense (anlamsız) mutasyon sebebiyle oluşan Duchenne tedavisine yönelik Ataluren (Translarna) ilacının FDA onay sürecine dair bir duyuru yayınladı. Not: İlaç, 2014'te EMA onayı almıştır. 5

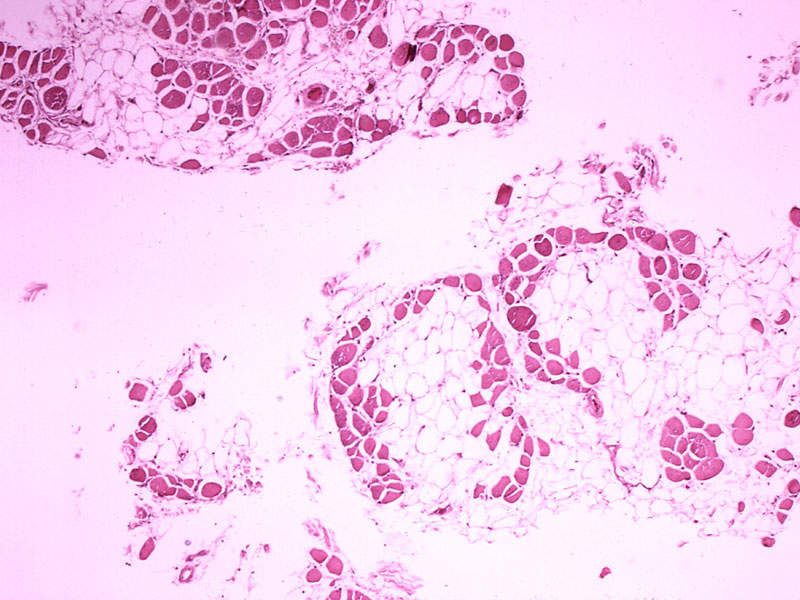
Frontiers | Off-Label Use of Ataluren in Four Non-ambulatory Patients With Duchenne Muscular Dystrophy: Effects on Cardiac and Pulmonary Function and Muscle Strength

Andy Biotech on Twitter: "EMA CHMP Jan Meeting Highlights http://t.co/0wgIFR8c6r Negative on $PTCT ataluren, $TEVA $ACTI.ST laquinimod, etc http://t.co/QvuSFipItp" / Twitter
PTC Therapeutics Announces CHMP Recommendation of Translarna™ (ataluren) Label Update for Non-Ambulatory Patients with Duchenn